Metacam Oral Suspension for Cats (Canada)
This treatment applies to the following species: Company: Boehringer Ingelheim Animal Health
Company: Boehringer Ingelheim Animal Health
Meloxicam 0.5 mg/mL
Veterinary Use Only
DIN 02360489
Description
Each mL contains 0.5 mg meloxicam in a yellowish suspension with an odour of honey and 1.5 mg of sodium benzoate (Ph. Eur.) as the preservative.
Metacam® Oral Suspension for Cats (0.5 mg/mL) is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam group. It acts by inhibition of prostaglandin synthesis, thereby exerting anti-inflammatory and analgesic effects.
Metacam Oral Suspension for Cats Indications
Metacam® Oral Suspension for Cats (meloxicam) is indicated for the alleviation of inflammation and pain (1) following surgery such as onychectomy, ovariohysterectomy or castration, or (2) associated with acute, mild to moderate musculoskeletal disorders in cats.
Dosage and Administration
Peri-operative use: After initial treatment with Metacam® 0.5% Injection for Dogs and Cats, continue treatment 24 hours later with Metacam® Oral Suspension for Cats 0.5 mg/mL at a dosage of 0.05 mg meloxicam/kg body weight. The oral follow-up dose may be administered once daily (at 24-hour intervals) for up to two days.
Acute musculoskeletal disorders: On the first day of treatment, a single oral dose of 0.1 mg meloxicam/kg body weight should be administered using Metacam® Oral Suspension for Cats. Treatment is to be continued once daily by oral administration (at 24 hour intervals) at a maintenance dose of 0.05 mg meloxicam/kg body weight for up to four days. Use the lowest effective dose for the shortest duration of treatment consistent with the individual clinical response.
Do not exceed the recommended dosage (see Contraindications and Cautions sections). Repeat dosing in cats exceeding the recommended dose or number of days of treatment has been associated with acute renal failure and death.
Owners should be advised when their cat has received a meloxicam injection, and be informed of the potential for adverse reactions and clinical signs associated with NSAID intolerance.
Always provide client information sheet with prescription.
Instructions for Use: Please carefully follow the instructions of the veterinarian. Particular care should be given with regard to the accuracy of dosing. Shake well before use.
To be administered orally either mixed with food or directly into the mouth. The suspension can be given using the drop dispenser of the bottle for cats of any body weight (0.017 mg/drop). Alternatively and for cats with a body weight of at least 2 kg, the Metacam® Oral Suspension for Cats measuring syringe (provided in the package) can be used.
Dosing procedure using the drop dispenser of the bottle:
Maintenance dose for peri-operative use: 3 drops/kg body weight.
First day dose for acute musculoskeletal disorders: 6 drops/kg body weight; subsequent daily maintenance dose: 3 drops/kg body weight.
Dosing procedure using the measuring syringe:
The syringe fits onto the drop dispenser of the bottle and has a kg-body weight scale which corresponds to the maintenance dose.
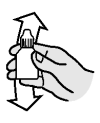
Shake bottle well. Push down and unscrew bottle top. Attach the dosing syringe to the drop dispenser of the bottle by gently pushing.
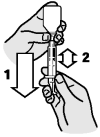
Turn the bottle/syringe upside down. Pull the plunger out until the black line on the plunger corresponds to your cat’s body weight in kilograms.
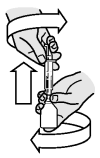
Turn the bottle right way up and with a twisting movement, separate the dosing syringe from the bottle.
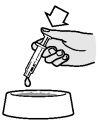
By pushing the plunger in, empty the contents of the syringe onto the food or directly into the mouth.
Use of the following bottle sizes after the first opening can be made as follows:
3 mL: Use within 14 days after the first opening.
15 mL: Use within 6 months after first opening.
Contraindications
Metacam® Oral Suspension for Cats (0.5 mg/mL) should not be administered if gastric or intestinal ulceration or bleeding is suspected; if there is evidence of cardiac, hepatic or renal disease; or if there is evidence of a haemorrhagic disorder or individual hypersensitivity to the product. Do not administer concurrently, other steroidal or nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycoside antibiotics or anticoagulant agents. Pre-treatment with other steroidal or nonsteroidal anti-inflammatory drugs (NSAIDs) may result in additional or increased adverse reactions and accordingly a treatment-free period with such drugs should be observed for at least 24 hours before commencement of treatment depending on the pharmacokinetic properties of the products used previously.
Do not use if there is evidence of dehydration, hypovolemia or hypotension, because of the increased risk of renal injury caused by the destruction of protective prostaglandins secreted by the kidneys in this risk situation.
Cautions: Particular care should be taken with regard to accuracy of dosing in cats. Do not exceed the stated dose. The accumulation of meloxicam occurs when an initial oral loading dose is given and followed by repeated oral dosing with Metacam® Oral Suspension for Cats.
The safety of Metacam has not been evaluated in breeding, pregnant or lactating cats. In case of overdosing, symptomatic treatment should be initiated. Increased risks of drug intolerance may occur in patients already debilitated. Animals being treated with meloxicam should be monitored for the occurrence of adverse reactions, as susceptibility varies with the individual.
All cats should undergo a thorough history and physical examination before the initiation of an NSAID treatment. When possible, appropriate laboratory tests should be conducted to establish hematological and biochemical baseline data before the administration of any NSAID.
If anorexia, lethargy, vomiting, diarrhea, increased drinking, increased or inappropriate urination or other suspected adverse reactions occur, IMMEDIATELY discontinue treatment and seek the advice of a veterinarian (see Adverse Reactions).
The use of Metacam® Oral Suspension for Cats less than 6 months of age or in debilitated aged animals may involve additional risk. If use in such animals cannot be avoided, a reduced dosage and careful clinical management may be required.
Warnings
- Keep out of reach of children.
- People with known hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs) should not handle this product.
- Pregnant women should not handle this product unless adequate exposure protection can be
assured.
- Caution should be taken to avoid accidental ingestion and contact with eyes.
- This product can cause eye irritation. In case of contact with the eyes, immediately rinse thoroughly with water.
Adverse Reactions
Typical adverse reactions of NSAIDs, such as loss of appetite, vomiting, diarrhea, apathy and polydipsia/polyuria associated with renal failure have occasionally been reported. These adverse effects are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal.
Although not all adverse reactions are reported, the following adverse reaction information is based on voluntary post-approval drug experience reporting by veterinarians and pet owners/caregivers. It should be noted that suspected adverse drug reactions listed here reflect reporting and not causality. The categories of adverse reactions are listed in decreasing order of frequency by body system. In rare cases, death has been associated with some of these adverse reactions.
Systemic disorders: anorexia, lethargy, death.
Digestive tract disorders: vomiting, diarrhea.
Renal and urinary disorders: renal disorder, renal failure, urine abnormalities. Repeat dosing in cats exceeding the recommended dose or number of days of treatment has been associated with acute renal failure and death.
Information for Cat Owners: Metacam® Oral Suspension for Cats (meloxicam) is a nonsteroidal anti-inflammatory drug (NSAID) and as with other drugs in this class, adverse reactions may occur in treated animals. The most common adverse reactions reported involve the kidneys and the gastrointestinal tract. Typical symptoms include loss of appetite, depression, vomiting, increased drinking, and increased or inappropriate urination. It is important in these situations to IMMEDIATELY discontinue treatment and contact your veterinarian. In most cases, the adverse reactions are transient and disappear after termination of treatment but in rare instances may be serious especially if treatment is not discontinued. Consult your veterinarian.
Pharmacokinetics: When Metacam® Oral Suspension for Cats is given in a fasted state, the maximal plasma concentrations are obtained after approximately 3 hours. If the cat is in a fed state at the time of dosing, absorption may be slightly delayed. Approximately 97% of meloxicam is bound to plasma proteins. Meloxicam is predominately found in plasma and is also a major biliary excretion product whereas urine contains only traces of parent compound.
Meloxicam is eliminated with a half-life of approximately 24 hours. Seventy-nine percent of the recovered dose is eliminated in the faeces and 21% in the urine. All major metabolites have been shown to be pharmacologically inactive. Administration of an initial oral loading dose of 0.1 mg/kg, followed by repeated oral dosing with Metacam® Oral Suspension for Cats at 0.05 mg/kg resulted in the accumulation of meloxicam with time (over 2 to 7 days).
Safety Studies: Studies on cats receiving meloxicam have demonstrated much higher sensitivity to NSAIDs than dogs. Cats receiving a subcutaneous dose of Metacam® 0.5% Injection at 0.3 mg/kg or 0.6 mg/kg, followed by the same dose orally with Metacam® 1.5 mg/mL Oral Suspension for 8 additional days (4 cats per group) had decreased appetites, acute gastrointestinal and circulatory disorders after day 7. Necropsy (day 9) confirmed treatment-related pyloric/duodenal ulceration and secondary peritonitis.
In a second study, cats received a subcutaneous dose of Metacam® 0.5% Injection at 0.3 mg/kg or 0.6 mg/kg, followed by 0.1 or 0.2 mg/kg orally for 9 additional days (four cats per group). One cat in each group was clinically depressed on the last day of treatment. Duodenal ulceration and secondary peritonitis were observed at necropsy.
A third study evaluated 1X, 3X and 5X subcutaneous injections (0.3 mg/kg) for 3 consecutive days (6 cats per group). Histopathology demonstrated slight to minimal papillary necrosis in the kidneys and mucosal erosion in the 3X and 5X groups. No treatment related changes were noted in the 1X group.
Note that, in the above studies, Metacam® 0.5% Injectable Solution and Metacam® 1.5 mg/mL Oral Suspension were used.
A fourth target animal safety study was carried out using Metacam® 0.5 mg/mL Oral Suspension for Cats. Adult cats (6 per group) received either placebo, 0.1 mg/kg day one followed by 0.05 mg/kg, 0.3 mg/kg day one followed by 0.15 mg/kg or 0.5 mg/kg day one followed by 0.25 mg/kg for 90 days. The only observed adverse reactions in the 1X group were vomiting and diarrhea in a single cat on five occasions on two separate days. The incidence of treatment-related gastrointestinal adverse reactions increased with increasing oral dosages and included vomiting, diarrhea and melenic feces. Evidence of gastrointestinal ulceration was apparent on histopathology in 1/6 cats in the 3X and 5X groups. The findings serve as a reminder of the sensitivity of cats to adverse reactions of NSAIDs and of the narrow therapeutic window of this group of drugs in the species.
A fifth tolerance study evaluated 14 days of treatment with either placebo, 0.025 (0.5X), 0.05 (1X) or 0.1 mg/kg (2X) the recommended dose (4 cats per group). No significant differences between groups were observed for any of the clinical, biochemical or pathological outcomes evaluated.
Efficacy clinical study for acute musculoskeletal disorders: The safety and clinical efficacy of Metacam® Oral Suspension for Cats (meloxicam) was evaluated in cats suffering from acute musculoskeletal disorders in a non-inferiority, positive-controlled, blinded, randomized, multi-centre field study.
In total, 120 cats (60 cats in each treatment group) were included in the study. Among the patients that completed the study, 54 cats were treated with Metacam® Oral Suspension for Cats at a dosage of 0.1 mg/kg once on the first day, followed by a dosage of 0.05 mg/kg once daily for the four following days, and 58 cats received the positive control drug at the approved dosage for 5 consecutive days.
The investigators conducted a clinical examination comprising general examination and specific examination of the musculoskeletal system at inclusion, on day 0 and at the end of treatment (day 5). These examinations included feed intake, general behaviour, posture, lameness and pain on manipulation assessments and rectal temperature measurement. In addition, at the end of the treatment, the investigator evaluated the overall efficacy and palatability of the treatment. Diseases of the limbs, especially soft tissue injuries (contusions) and joint disorders (sprains and luxations) of less than 2 weeks duration were the most frequently diagnosed.
The primary variable to evaluate the efficacy of Metacam® Oral Suspension for Cats was the change from baseline of the Clinical Sum Score (CSS) calculated by adding the single scores of posture, lameness and pain on manipulation. Change from baseline was -4.3 in the Metacam® Oral Suspension for Cats group and -4.4 in the positive control group. For this primary variable, significant non-inferiority of Metacam® Oral Suspension for Cats in comparison to the positive control was shown and thus proved efficacy.
For each single parameter of the CSS (posture, lameness, and pain on manipulation), as well as the secondary parameters (feed intake, general behavior, and rectal temperature), the differences between the two groups were very small and no statistically significant difference was found between the two treatments.
However, the palatability of Metacam® Oral Suspension for Cats was found to be significantly better (p< 0.001) than the palatability of the positive control.
Adverse events were reported for 3 cats treated with Metacam® Oral Suspension for Cats with none of these classified as probably related to the treatment. In the positive control group, adverse events were reported in 3 cats, including two classified as probably related to the treatment.
Metacam® Oral Suspension for Cats, at a starting dosage of 0.1 mg/kg once on the first day of treatment followed by a dosage of 0.05 mg/kg once daily for four consecutive days in cats suffering from acute musculoskeletal disorder, proved to be efficacious, safe and easy to administer.
Storage
Store at or below 25°C.Presentation: Metacam® Oral Suspension for Cats is supplied in 3 and 15 mL bottles, containing 0.5 mg/mL of meloxicam.
*References available upon request.
METACAM® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
Boehringer Ingelheim Animal Health Canada Inc., 5180 South Service Road, Burlington ON L7L 5H4
Revised: 11-2022
159628-005
CPN: 1182188.0
5180 SOUTH SERVICE ROAD, BURLINGTON, ON, L7L 5H4
| Customer Care No.: | 1-800-567-1885 | |
| Technical Services No.: | 1-877-565-5501 | |
| Website: | www.boehringer-ingelheim.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
