Domidine 10 mg/mL (Canada)
This treatment applies to the following species: Company: Dechra
Company: Dechra
Detomidine hydrochloride injection
Veterinary Use Only
DIN 02485303
Sterile - 10 mg/mL
Sedative and analgesic for horses.
Description
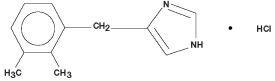
Domidine is a synthetic α2-adrenoceptor agonist with sedative and analgesic properties. The chemical name is 1H-imidazole, 4-[(2,3-dimethylphenyl)methyl]-hydrochloride and the generic name is detomidine hydrochloride. It is a white, crystalline, water-soluble substance having a molecular weight of 222.7. The molecular formula is C12H14N2•HCl.
Chemical Structure:
Active Ingredients
Each mL contains 10 mg detomidine hydrochloride.
Preservative: Contains 1.0 mg/mL methylparaben.
Domidine 10 mg/mL Indications
For use as a sedative and analgesic to facilitate minor surgical and diagnostic procedures in mature horses and yearlings.
Dosage and Administration
Administer Domidine IV or IM at the rates of 20 or 40 µg detomidine hydrochloride per kg of body weight (0.2 to 0.4 mL of Domidine per 100 kg or 220 lbs.), depending on the depth and duration of sedation and analgesia required. Analgesia is more pronounced when the drug is given IV. Sedative and analgesic effects will usually occur in 2 to 5 minutes and will persist for 30 minutes to 2 hours, depending on dosage and route of administration.Prior to and following injection, the animal should be allowed to rest quietly. The full effect should be reached within 2 to 4 minutes after IV injection and 3 to 5 minutes after IM injection.
Contraindications
Not to be used in horses with pre-existing AV or SA block, with severe coronary insufficiency, cerebrovascular disease, respiratory disease, or chronic renal failure. Detomidine hydrochloride should not be used in breeding horses since the potential risk has not been assessed in either stallions or mares.
CAUTIONS: The drug is a potent α2-agonist which should be used with extreme caution with other sedatives, analgesics, or anesthetics as these are likely to produce additive effects. Careful consideration should be given prior to the administration of Domidine to horses in endotoxic or traumatic shock or approaching shock, to horses with advanced liver or kidney disease or other pre-existing conditions of stress such as extreme heat or cold, high altitude, or fatigue. Intravenous potentiated sulfonamides should not be used in anesthetized or sedated horses as potentially fatal dysrhythmias may occur. Protect treated horses from temperature extremes. Food and water should be withheld until the sedative effect of Domidine has worn off.
Warnings
Keep out of reach of children. Not for use in horses that are to be slaughtered for use in food.Adverse Reactions
As with all α2-agonists, the potential for isolated cases of hypersensitivity, including paradoxical response (excitation) exists. There has been an occasional appearance of arrhythmia, hypertension, and bradycardia in horses treated with detomidine hydrochloride injection. Piloerection, sweating and salivation, and, occasionally, slight muscle tremors are frequently seen after administration. Partial transient penis prolapse may be seen. Partial atrioventricular and sino-auricular blocks may occur with decreased heart and respiratory rates. Urination typically occurs during recovery at about 45 to 60 minutes post-treatment, depending on dosage. Incoordination or staggering is usually seen only during the first 3 to 5 minutes after injection, until animals have secured a firm footing. Because of continued lowering of the head during sedation, passive congestion may occur occasionally in the lips, the facial area, and the upper airways. Tying the head in a slightly elevated position generally prevents these effects.Post-approval experience: Although all adverse reactions are not reported, the following adverse reaction information is based on voluntary post-approval drug experience reporting. It is generally recognized that this method of reporting results in a significant under-reporting of adverse drug reactions. It should also be noted that suspected adverse drug reactions listed here reflect reporting and not causality. The categories of adverse reactions are listed in decreasing order of frequency by body system.
Respiratory tract disorders: hyperventilation
Immune system disorders: urticaria, hypersensitivity
Neurological disorders: profound sedation, ataxia
Systemic disorders: hyperthermia, lack of efficacy
NOTE TO USERS: Some horses, although apparently deeply sedated, may still respond to external stimuli. Routine safety measures should be employed to protect practitioners and handlers. Allowing the horse to stand quietly for 5 minutes prior to and after injection may improve the response to Domidine.
Clinical Pharmacology
Domidine, a non-narcotic sedative and analgesic, is a potent α2-adrenoceptor agonist which produces sedation and superficial and visceral analgesia which is dose dependent in its depth and duration. Profound lethargy and a characteristic lowering of the head with reduced sensitivity to environmental stimuli (sounds, etc.) are seen with detomidine. A short period of incoordination is characteristically followed by immobility and a firm stance with front legs well spread. The analgesic effect is most readily seen as an increase in the pain threshold at the body surface. With IV administration, both superficial and visceral analgesia are seen to depths which allow for such procedures as flank incision after local anesthesia, penetration of the peritoneum, and rectal examinations for diagnosis of colic. Sensitivity to touch is little affected and in some cases may actually be enhanced.Chemical restraint and pain relief provided by α2-adrenoceptors facilitates handling of fractious animals, and aids in the conduct of uncomfortable diagnostic or therapeutic procedures such as endoscopy, nasogastric tubing, and rectal examinations. It also facilitates surgical procedures (with or without local anesthesia) such as suturing of skin lacerations, flank incisions for ovariectomy, vaginal suturing, and castration.
With detomidine administration, heart rate is markedly decreased, blood pressure is initially elevated and then a steady decline to normal is seen. A transient change in the conductivity of the cardiac muscle may occur, as evidenced by partial atrioventricular (AV) and sino-auricular (SA) blocks. This change in the conductivity of the cardiac muscle may be prevented by IV administration of atropine at 0.012 mg/kg of body weight.
A diuretic effect is usually observed within 45 to 60 minutes after treatment. No effect on blood clotting time or other hematological parameters was encountered at dosages of 20 or 40 µg/kg of body weight. Respiratory responses include an initial slowing of respiration within a few seconds to 1 to 2 minutes after administration, increasing to normal within 5 minutes. An initial decrease in tidal volume is followed by an increase.
SAFETY STUDIES: Detomidine hydrochloride is tolerated in horses at up to 200 µg/kg of body weight (10 times the low dosage and 5 times the high dosage). In safety studies in horses, detomidine hydrochloride at 400 µg/kg of body weight administered daily for 3 consecutive days produced microscopic foci of myocardial necrosis in 1 of 8 horses.
Storage
Store between 15 and 30°C. Contents should be used within 28 days after first use.PRESENTATION: Domidine is supplied in 10 and 20 mL multidose vials.
Eurovet Animal Health B.V., Bladel, The Netherlands
Imported and distributed by: Dechra Veterinary Products Inc., 1 Holiday Avenue, East Tower, Suite 345, Pointe-Claire, QC H9R 5N3
®2016 Dechra Ltd. Domidine is a registered trademark of Dechra Ltd. All rights reserved.
Lot/EXP: See bottom of carton.
617306
|
|
GTIN: |
|
|
10 mL |
05701170344929 |
617305 |
|
20 mL |
05701170344936 |
617310 |
CPN: 1786049.0
1 HOLIDAY AVE., EAST TOWER SUITE 345, POINT-CLAIRE, QC, H9R 5N3
| Toll-Free: | 855-332-9334 | |
| Technical Services: | 855-332-9334 Option 1 | |
| Technical Services Email: | technical.ca@dechra.com | |
| Website: | www.dechra.ca |
 |
THIS SERVICE AND DATA ARE PROVIDED "AS IS". Animalytix assumes no liability, and each user assumes full risk, responsibility, and liability, related to its use of the Animalytix service and data. See the Terms of Use for further details. |

Copyright © 2024 Animalytix LLC. Updated: 2024-02-27
