Clindamycin Topical Prescribing Information
Package insert / product label
Generic name: clindamycin phosphate
Dosage form: topical gel
Drug classes: Topical acne agents, Vaginal anti-infectives
Medically reviewed by Drugs.com. Last updated on Jun 7, 2024.
On This Page
Clindamycin Topical Description
Clindamycin phosphate gel USP, 1%, a topical antibiotic, contains clindamycin phosphate, USP, at a concentration equivalent to 10 mg clindamycin per gram in a gel vehicle consisting of propylene glycol, polyethylene glycol 400, methylparaben, carbomer homopolymer type A, sodium hydroxide and purified water. Chemically, clindamycin phosphate, USP is a water-soluble ester of the semi-synthetic antibiotic produced by a 7 (S)-chloro-substitution of the 7 (R)-hydroxyl group of the parent antibiotic, lincomycin, and has the structural formula represented below:
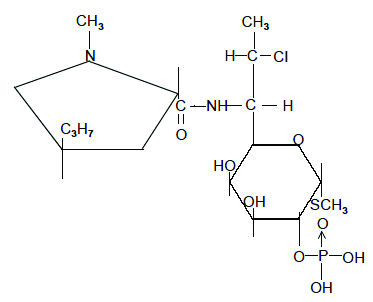
The chemical name for clindamycin phosphate, USP is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate).
Clindamycin Topical - Clinical Pharmacology
Pharmacokinetics
In an open label, parallel group study of 24 patients with acne vulgaris, once-daily topical administration of approximately 3 to 12 grams/day of clindamycin phosphate gel for five days resulted in peak plasma clindamycin concentrations that were less than 5.5 ng/ml.
Following multiple applications of clindamycin phosphate gel less than 0.04% of the total dose was excreted in the urine.
Microbiology
Although clindamycin phosphate is inactive in vitro, rapid in vitro hydrolysis converts this compound to clindamycin which has antibacterial activity. Clindamycin inhibits bacteria protein synthesis at the ribosomal level by binding to the 50S ribosomal subunit and affecting the process of peptide chain initiation. In vitro studies indicated that clindamycin inhibited all tested Propionibacterium acnes cultures at a minimum inhibitory concentration (MIC) of 0.4 mcg/ml. Cross-resistance has been demonstrated between clindamycin and erythromycin.
Related/similar drugs
doxycycline, metronidazole, metronidazole topical, clindamycin, erythromycin topical, tetracycline, isotretinoin
Clinical Studies
In one 12-week, multicenter, randomized, evaluator-blind, vehicle-controlled, parallel comparison clinical trial in which patients used clindamycin phosphate gel, 1% once daily or the vehicle gel once daily, in the treatment of acne vulgaris of mild to moderate severity, clindamycin phosphate gel applied once daily was more effective than the vehicle applied once daily. The mean percent reductions in lesion counts at the end of treatment in this study are shown in the following table:
|
Lesions |
Clindamycin Phosphate Gel |
Vehicle Gel |
|
Inflammatory |
51% |
40% * |
|
Noninflammatory |
25% |
12% * |
|
Total |
38% |
27% * |
|
*P< 0.05 |
||
There was a trend in the investigator’s global assessment of the results which favored clindamycin phosphate gel QD over the vehicle QD.
In a contact sensitization study, four of the 200 subjects appeared to develop suggestive evidence of allergic contact sensitization to clindamycin phosphate gel. There was no signal for contact sensitization in the clinical trials under normal use conditions.
Indications and Usage for Clindamycin Topical
Clindamycin phosphate gel, 1% is indicated for topical application in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician should consider whether other agents are more appropriate (see CONTRAINDICATIONS, WARNINGS, and ADVERSE REACTIONS).
Contraindications
Clindamycin phosphate gel is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional enteritis or ulcerative colitis, or a history of antibiotic-associated colitis.
Warnings
Orally and parenterally administered clindamycin has been associated with severe colitis, which may result in patient death. Use of the topical formulation of clindamycin results in absorption of the antibiotic from the skin surface. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin.
Studies indicate a toxin(s) produced by Clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Endoscopic examination may reveal pseudomembranous colitis. Stool culture for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
When significant diarrhea occurs, the drug should be discontinued. Large bowel endoscopy should be considered to establish a definitive diagnosis in cases of severe diarrhea. Antiperistaltic agents, such as opiates and diphenoxylate with atropine, may prolong and/or worsen the condition.
Diarrhea, colitis, and pseudomembranous colitis have been observed to begin up to several weeks following cessation of oral and parenteral therapy with clindamycin.
Precautions
Drug Interactions
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity of a 1% clindamycin phosphate gel similar to clindamycin phosphate gel was evaluated by daily application to mice for two years. The daily doses used in this study were approximately 3 and 15 times higher than the human dose of clindamycin phosphate from 5 milliliters of clindamycin phosphate gel, assuming complete absorption and based on a body surface area comparison. No significant increase in tumors was noted in the treated animals.
A 1% clindamycin phosphate gel similar to clindamycin phosphate gel caused a statistically significant shortening of the median time to tumor onset in a study in hairless mice in which tumors were induced by exposure to simulated sunlight.
Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
Reproduction studies in rats using oral doses of clindamycin hydrochloride and clindamycin palmitate hydrochloride have revealed no evidence of impaired fertility.
Pregnancy: Teratogenic effects
Reproduction studies have been performed in rats and mice using subcutaneous and oral doses of clindamycin phosphate, clindamycin hydrochloride and clindamycin palmitate hydrochloride. These studies revealed no evidence of fetal harm. The highest dose used in the rat and mouse teratogenicity studies was equivalent to a clindamycin phosphate dose of 432 mg/kg. For a rat, this dose is 84 fold higher, and for a mouse 42 fold higher, than the anticipated human dose of clindamycin phosphate from clindamycin phosphate gel based on a mg/m2 comparison. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether clindamycin is excreted in human milk following use of clindamycin phosphate gel. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Adverse Reactions/Side Effects
In the one well-controlled clinical study comparing clindamycin phosphate gel and its vehicle, the incidence of skin and appendages adverse events occurring in ≥1% of the patients in either group is presented below:
|
Number (%) of Patients |
||
|
Body System/Adverse Event |
Clindamycin Phosphate Gel |
Vehicle Gel |
|
Skin and appendages disorders | ||
|
Dermatitis |
0 (0.0) |
1 (1.2) |
|
Dermatitis contact |
0 (0.0) |
1 (1.2) |
|
Dermatitis fungal |
0 (0.0) |
1 (1.2) |
|
Folliculitis |
0 (0.0) |
1 (1.2) |
|
Photosensitivity reaction |
0 (0.0) |
1 (1.2) |
|
Pruritus |
1 (0.6) |
1 (1.2) |
|
Rash erythematous |
0 (0.0) |
0 (0.0) |
|
Skin dry |
0 (0.0) |
0 (0.0) |
|
Peeling |
1 (0.6) |
0 (0.0) |
Orally and parenterally administered clindamycin has been associated with severe colitis, which may end fatally.
Cases of diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported as adverse reactions in patients treated with oral and parenteral formulations of clindamycin and rarely with topical clindamycin (see WARNINGS). Abdominal pain and gastrointestinal disturbances, as well as gram-negative folliculitis, have also been reported in association with the use of topical formulations of clindamycin.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
Topically applied clindamycin phosphate gel may be absorbed in sufficient amounts to produce systemic effects (see WARNINGS).
Clindamycin Topical Dosage and Administration
Apply a thin film of clindamycin phosphate gel, 1% once daily to the skin where acne lesions appear. Use enough to cover the entire affected area lightly.
Keep container tightly closed.
How is Clindamycin Topical supplied
Clindamycin phosphate gel USP, 1% containing clindamycin phosphate, USP equivalent to 10 mg clindamycin per gram, is available in the following size:
75 mL bottle - NDC 69238-2031-7
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Do not store in direct sunlight.
CAUTION
Federal law prohibits dispensing without prescription.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 04-2021-00
| CLINDAMYCIN PHOSPHATE
clindamycin phosphate gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amneal Pharmaceuticals NY LLC (123797875) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amneal Pharmaceuticals, LLC | 079389286 | analysis(69238-2031) , label(69238-2031) , manufacture(69238-2031) , pack(69238-2031) | |
More about clindamycin topical
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (231)
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical acne agents
- Breastfeeding
Patient resources
Professional resources
- Clindamycin (Topical) monograph
- Clindacin Foam (FDA)
- Clindamycin Foam (FDA)
- Clindamycin Gel (FDA)
- Clindamycin Lotion (FDA)
Other brands
Clindagel, Clindesse, Xaciato, Clindamax, ... +4 more


