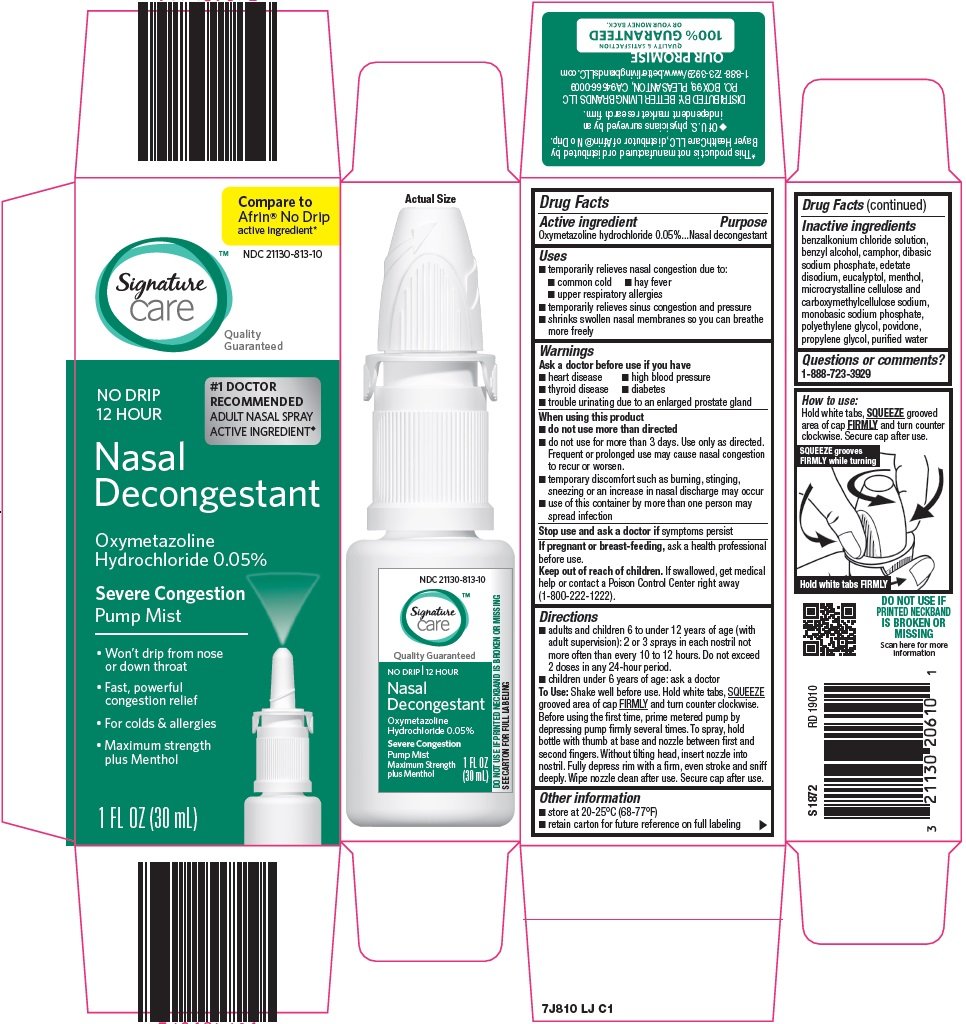
signature care nasal decongestant
Dosage form: spray
Ingredients: OXYMETAZOLINE HYDROCHLORIDE 0.05g in 100mL
Labeler: Safeway
NDC code: 21130-813
Medically reviewed by Drugs.com. Last updated on May 25, 2023.
Oxymetazoline hydrochloride 0.05%
Nasal decongestant
- •
- temporarily relieves nasal congestion due to:
- •
- common cold
- •
- hay fever
- •
- upper respiratory allergies
- •
- temporarily relieves sinus congestion and pressure
- •
- shrinks swollen nasal membranes so you can breathe more freely
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- do not use more than directed
- •
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- •
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- •
- use of this container by more than one person may spread infection
symptoms persist
ask a health professional before use.
If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
- •
- adults and children 6 to under 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- •
- children under 6 years of age: ask a doctor
To Use: Shake well before use. Hold white tabs, SQUEEZE grooved area of cap FIRMLY and turn counter clockwise. Before using the first time, prime metered pump by depressing pump firmly several times. To spray, hold bottle with thumb at base and nozzle between first and second fingers. Without tilting head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and sniff deeply. Wipe nozzle clean after use. Secure cap after use.
- •
- store at 20-25°C (68-77°F)
- •
- retain carton for future reference on full labeling
benzalkonium chloride solution, benzyl alcohol, camphor, dibasic sodium phosphate, edetate disodium, eucalyptol, menthol, microcrystalline cellulose and carboxymethylcellulose sodium, monobasic sodium phosphate, polyethylene glycol, povidone, propylene glycol, purified water
1-888-723-3929
Compare to Afrin® No Drip active ingredient
Quality Guaranteed
NO DRIP 12 HOUR
#1 DOCTOR RECOMMENDED ADULT NASAL SPRAY ACTIVE INGREDIENT
Nasal Decongestant
Oxymetazoline Hydrochloride 0.05%
Severe Congestion
Pump Mist
Won’t drip from nose or down throat
Fast, powerful congestion relief
For colds & allergies
Maximum Strength plus Menthol
1 FL OZ (30 mL)
| SIGNATURE CARE NASAL DECONGESTANT
oxymetazoline hydrochloride spray |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Safeway (009137209) |
Further information
Always consult your healthcare provider to ensure the information displayed on this page applies to your personal circumstances.